Copy. Paste. Profit.

You pick up your prescription.
Your doctor wrote for a brand-name drug, but your insurance didn’t cover it.
So the pharmacist says: “We’ll give you the generic instead. Trust me. It’s the same thing.”
You nod. It’s all good. You’re used to this song and dance.
You get home, open the bottle... and you do a double-take.
The pill inside looks identical to the brand-name version you had before. Same shape. Same size. Same markings. Same color. In fact, it was made by the same manufacturer.
Well, that’s because it is the same pill. Literally.
You saved $100 by switching the label on the bottle—not the drug. And somewhere, a pharmaceutical company made a whole lot more.
Welcome to the strange world of authorized generics—Big Pharma market manipulation disguised as competition.

💊 So… What is an Authorized Generic?
The term “generic” usually means a cheaper version of a drug made by a different manufacturer after a brand-name patent expires. It has the same active ingredient, but the fillers and inactive ingredients might differ. Generics must meet strict FDA bioequivalence standards, and when multiple generics enter the market, prices typically drop fast.
But an Authorized Generic is something very different.
It’s when the brand-name manufacturer themselves sells their own drug in a generic bottle—often during the 180-day exclusivity window right after the patent expires, when only one other generic is legally allowed to compete.
They don’t change a thing. They just strip the branding, slap on a new label, and sell it through a subsidiary or third party.
Same formulation. Same factory. Same pill.
From the FDA’s own website:

They don’t even try to sugar-coat it!
Here’s how it actually plays out…

💵 A Real-World Example: Adderall XR
Let’s rewind to 2009. You’re taking Adderall XR, the popular extended-release stimulant for ADHD, made by Shire.
The patent’s about to expire. A generic manufacturer—Teva—files an application to make a cheaper version. The FDA grants them 180 days of exclusivity, a legal reward for being the first to challenge the patent.
But instead of fighting it out, Shire cuts a deal.
Teva agrees to delay launching its own version of Adderall XR—and in exchange, Shire authorizes Teva to sell the exact same drug under a “generic” label. Same pill. Same factory. Same everything. They split the profits.
So now, during the exclusivity period, there are technically two generics on the market:
Teva’s authorized generic (really just brand Adderall XR in a different bottle)
Teva’s “true” generic (which they conveniently delay 180 days until after they milk the exclusive profits from the authorized version)
And no other generics are allowed to enter.
Teva gets the monopoly profits. Shire keeps manufacturing volume. And you, the consumer, get a high-cost “generic” that isn’t generic at all.
The price drops slightly, but not by much. There’s still no real competition. Just a clever workaround.
You might be thinking: “Okay, but it’s just six months. How much impact can it really have?”
Per Health Affairs:
In a 2013 opinion, the US Supreme Court noted that the 180-day exclusivity period can be worth “several hundred million dollars” to generic manufacturers.
Cha-Ching! Here’s my weekly reminder that Healthcare is a Business!

Let’s break down how this benefits the manufacturer
Imagine the branded version of a drug costs $500 per month.
A typical generic, once there’s full competition, might cost $50 or even less.
But during the 180-day exclusivity period, a generic manufacturer might price their version at $400—just under the brand medication. Still a huge profit.
If the brand manufacturer plays ball with an authorized generic of its own drug, it gets to:
Recapture market share under a different label
Undercut the “true” generic on price
Avoid losing volume or revenue—because they’re still the ones making the drug
Meanwhile, patients and payers are happy to pay $400 instead of $500, even though an honest open market would have pushed the price way lower.
For arguments sake:
Brand drug (original): $500
True generic competitor: $400
Authorized generic (made by brand): $390
Patients switch to the $390 option.
The “brand” company still makes the product.
The insurer gets to check a box that they saved money.
And generic competition is blunted, if not entirely blocked.

🧪 Other Real World Examples of Authorized Generics
EpiPen
After massive public backlash over its $600+ price tag, Mylan launched an “authorized generic” version of EpiPen in 2016. It was the exact same auto-injector, just without the branding.
The authorized generic retailed for ~$300. Still expensive. But it helped Mylan quiet criticism without actually introducing new competition.

Look familiar?

It’s the same thing!
Advair Diskus
For years, GlaxoSmithKline (GSK) sold its blockbuster asthma medication Advair Diskus without a generic. When generics were finally approved, GSK began offering an authorized generic through another manufacturer, Prasco, to maintain control of the pipeline. Below are real pictures I took yesterday of the two packages side-by-side in my own pharmacy. They barely bother changing the design! I left the price stickers on for you. Comical.

A simple photoshop job works wonders!

$234.94 vs $47.40 for the same medication! (Some info redacted for legal reasons)
Dexilant
This is one of my favorite examples. Unfortunately, I didn’t have a bottle of brand name Dexilant in stock to take a picture of, but the pictures below tell enough of the story. First is a bottle of the authorized generic and its price-per-pill from my wholesaler. Underneath is a screenshot of the brand name price and pill that I also pulled from a drug wholesalers website today. Same capsule. Same factory. More than twice the price. Isn’t pharma the best?!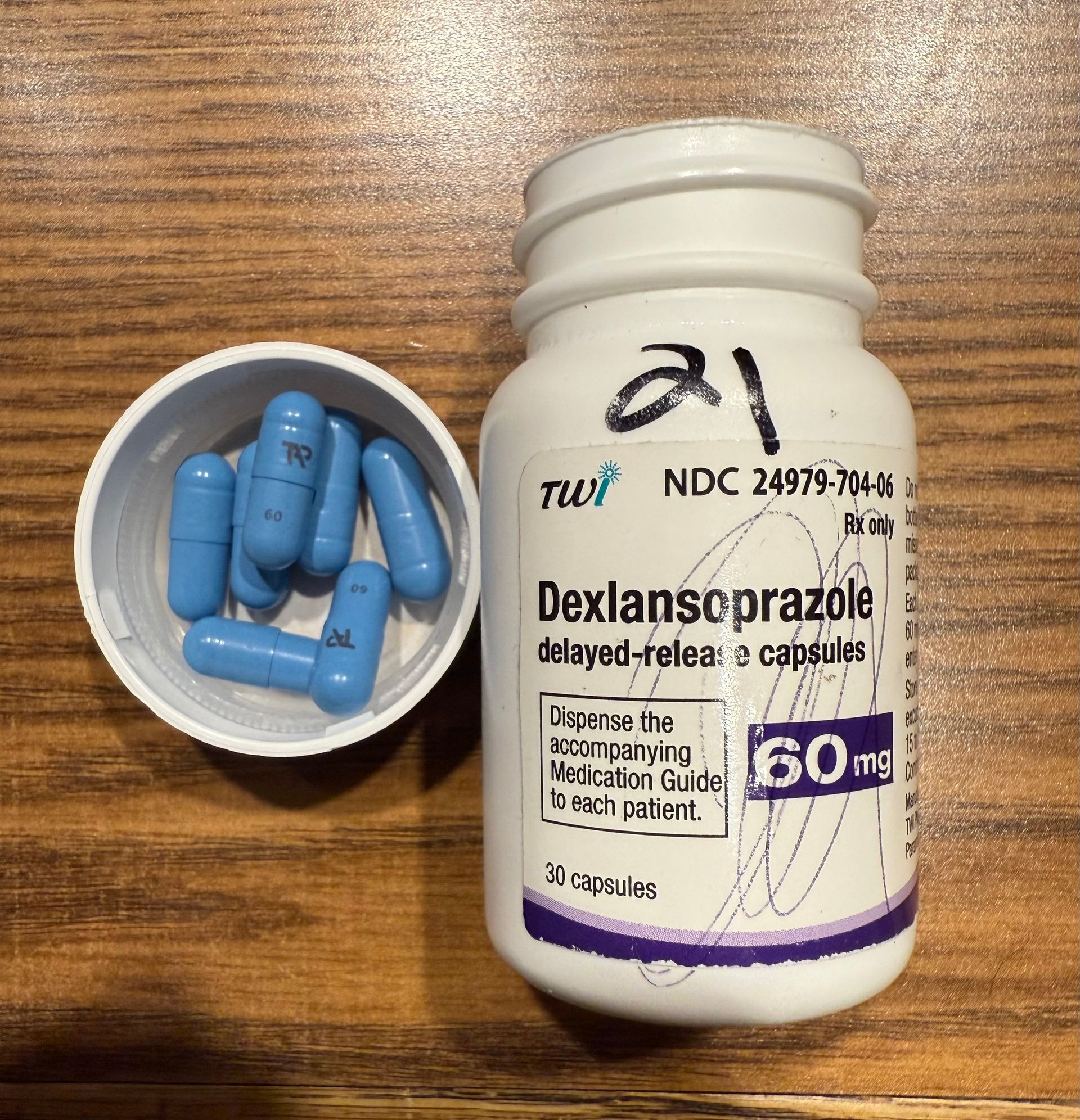
I took this picture myself from a bottle on the shelf of my pharmacy yesterday.

$4.521 per pill from the bottle above.
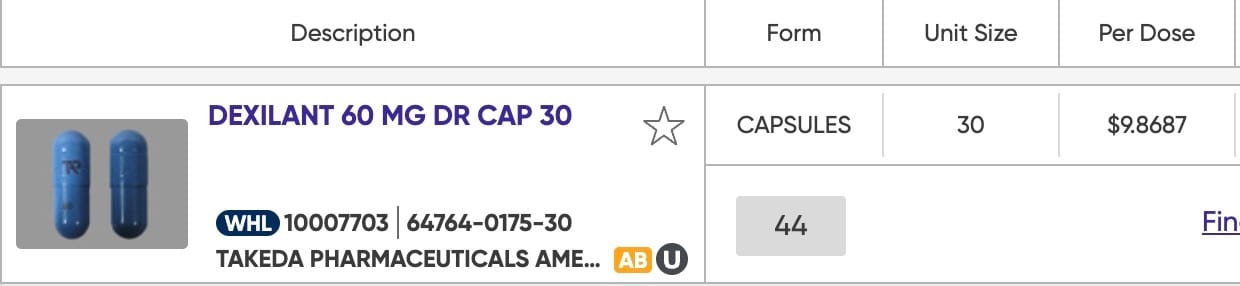
$9.8687 per pill for this brand name Dexilant. Notice the pic of the pill matches exactly what is in the bottle above.

🥊 Why This Matters
Authorized generics aren’t always bad. In some cases, they do bring prices down in the short term—especially if the branded company is responding to competition they can’t stop.
But more often than not, they serve as a strategic shield:
To preserve profits
To extend monopoly power
To undermine generic entrants
To create the illusion of competition
It’s drug pricing theater.
And we’re all in the audience paying the absurd admission fee.

🧠 Why It’s Allowed
The system lets it happen because it technically increases competition. The FDA doesn’t regulate pricing—it only regulates safety, efficacy, and labeling. And payers, PBMs, and manufacturers are all financially incentivized to keep that separation intact. So as long as the authorized generic is accurately labeled and meets quality standards, it’s legal.
And from a business standpoint, it’s a win:
The brand drug keeps revenue flowing.
The authorized “generic” enters the market without risk of stealing profit.
The PBM still gets rebates (sometimes from both sides).
Patients save a little—but not as much as they should.
The losers?
Us.

The Drugstore Cowboy Take
Authorized generics are the kind of quiet scam that define American pharmacy.
They aren’t technically scams. They follow the rules. They save you a little money. They sound good in earnings calls. But make no mistake—this system was designed by people with MBAs, not MDs.
It’s the same story I’ve told here week after week. Every big company in healthcare has figured out exactly how close they can get to the line without crossing it. And every inch closer they get multiplies their profits exponentially.
When the same company is selling two versions of the exact same drug at two very different prices, we should be asking the same questions I leave you with every week…
Why is this allowed?
Who profits?
And how do we fix it?
Until we overhaul the incentive structure, these tricks will keep happening.
I don’t know about you, but I’m tired of watching the magic show. Once you know how the illusion works, it never hits the same again.
We don’t need magic. We need transparency.
And maybe a refund.
Keep your eyes on the horizon. The sun will rise soon enough.

Alec Wade Ginsberg, PharmD, RPh
4th-Gen Pharmacist | Owner & COO, C.O. Bigelow
Founder, Drugstore Cowboy
