How a Drug Becomes a Drug

A few days ago I scrolled past a headline in the Wall Street Journal that made me stop what I was doing and read the full article immediately.

Here’s the gist:
In May, the state of Montana significantly broadened its “Right to Try” laws by passing new legislation that lets clinics provide treatments still awaiting FDA approval. Under the new rules, licensed experimental clinics are permitted to recommend and administer drugs that have only completed Phase I trials—the earliest stage of human testing, focused on safety rather than effectiveness.
Thanks to this change, the state is bracing for a gold rush of loosely regulated clinics. It’s basically a supercharged combination of what I’ve recently written about supplements and advertising. Montana is about to become a hub for medical tourism—offering access to unproven, experimental treatments still working their way through the FDA approval process.
They’ll be expensive.
They’ll be aggressively marketed.
And you better believe patients are going to show up.
It sounds liberating. American. The rugged frontier of medical innovation.
But there’s a reason we usually wait.
Because bringing a drug to market is hard. It’s slow. It’s expensive.
And when shortcuts are taken—it’s dangerous.
There are many angles to this story, and I could write 5,000 words on each. But what struck me most was this: most people have no idea what it actually takes to bring a drug from idea to shelf.
And as a healthcare consumer, that’s important.
So today, we’re going back to basics:
How does a drug become a drug?
What does it cost?
How long does it take?
And what happens when people try to skip the line?

🚧 The Long Road to Approval
Let’s start at the beginning—before the Montana clinics, before the pharmacy, before the TV ad that says “ask your doctor.”
This is the real process:
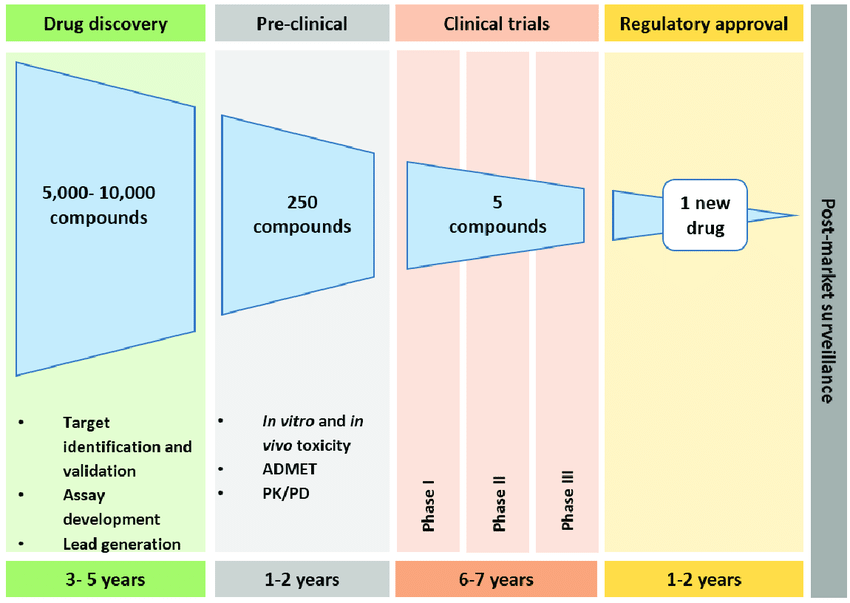
1. Drug Discovery & Preclinical Testing
🕒 Timeframe: 3–7 years
Researchers identify a target—a protein, receptor, or biological process involved in disease.
Thousands of chemical compounds are screened to see which ones might interact with it.
Promising candidates go through animal studies and lab tests to evaluate toxicity and basic efficacy.
💥 Most drugs fail here.
Out of 5,000–10,000 compounds screened, only about 250 make it to preclinical testing. Of those, only 5 might move forward to human trials.【source】

2. Clinical Trials
🕒 Timeframe: 6–7 years
If early data looks promising, the drug sponsor files an Investigational New Drug (IND) application with the FDA to begin human testing.
Clinical trials happen in three phases:
Phase I (Safety): 20–100 healthy volunteers. Is it safe? What’s the right dose?
Phase II (Efficacy): 100–500 patients. Does it work? What are the side effects?
Phase III (Comparison): 1,000+ patients. Is it better than what’s already on the market?
🧪 Only 12% of drugs that enter clinical trials are ever approved by the FDA.
These trials often span multiple years, cost hundreds of millions of dollars, and require constant FDA oversight.
One bad outcome can derail the entire program.

3. FDA Review & Approval
🕒 Timeframe: 1–2 years
Once trials are complete, the company submits a New Drug Application (NDA) or Biologics License Application (BLA) to the FDA. These submissions can exceed 100,000 pages.
The FDA:
Reviews safety, efficacy, labeling, and manufacturing processes
Convenes advisory committees to vote on approval
Inspects the company’s manufacturing facilities
If all goes well, the drug is approved.
And only then can your doctor prescribe it.
This is the level of scrutiny you’d expect before giving a drug to your parents, your partner, or your child.

💰 What Does This Cost?
According to the Tufts Center for the Study of Drug Development, the average cost to develop a new drug—including failures—is about $2.6 billion【source】.
Other estimates range from $1–$2 billion, depending on methodology. But the key point is this:
You’re not just paying for the drug that worked.
You’re paying for the graveyard of drugs that didn’t.

Where does the money go?
R&D costs: Scientists, lab equipment, testing
Opportunity costs: A decade of sunk time and capital
Manufacturing & compliance: Especially for complex biologics
Marketing & legal: Sales reps, TV ads, insurance risk

🧬 Why Do Drug Companies Get Patents?
Because no one would spend billions of dollars and over a decade of time developing a new drug… only to have a competitor knock it off the day it hits the market.
Here’s how it works:
Drug patents are usually filed early in the development process, before trials even begin
Patents last 20 years from the date of filing
By the time the drug is approved, there are often 8–12 years of protection remaining
That’s the window where the company tries to recoup its investment—and make a profit.
After that, generic manufacturers can enter the market.
When they do, brand-name drug sales are reduced to basically nothing. That period of exclusivity is worth everything.

🧠 Innovation Needs Incentive
This isn’t a defense of $26,000-a-year Wegovy.
But it is a defense of the basic idea that drug development only happens when there’s a financial incentive.
I’ve said it before, and I’ll say it again:
If you want more cures, someone has to fund the failures, the trials, and the teams of scientists doing the work.
We may disagree on how those profits are made—but the need for a profit window is non-negotiable.
⚖️ What’s the Risk of Skipping the Line?
When we bypass the traditional development process, we also bypass:
Safety checks
Dosing protocols
Long-term monitoring
Data collection
Which means when something goes wrong…
No one knows why.
I believe patients with terminal illnesses should be allowed to try experimental treatments. That’s the original intent of “Right to Try.”
But what’s happening in Montana is something else entirely.
These laws are now being exploited by operators more interested in profit than protocols.
This is what we’re looking at very soon:
You see a targeted ad on TikTok:
“Our clinic has the next generation of Ozempic! It won’t be available anywhere else in the United States for 8 years! Only $12,000 per month!”
How cool! You can get next generation Ozempic before the FDA approves it? Your friends will be so jealous! You fly to the clinic to try it. They hand you a multi-page waiver. You don’t have the patience to read it, and the clinic looks legit, so you sign. You just want to lose weight as fast as you can. You can’t wait.
You hand over your credit card.
A couple months later, you realize this drug doesn’t actually work. And it actually makes you feel some side effects. You’ve already spent over $50,000.
You see in the news that the Phase II trials didn’t prove enough for the drug to continue down the pipeline. It doesn’t actually work. You feel robbed! How could this happen?
You call the clinic. They say “you already signed away liability. That part was in the fine print.”
This is the new reality. And in that example, you’re just out a bunch of money. There will be plenty of cases where the side effects are more than just a nuisance (and the meds are more affordable). This is a very slippery slope.

🧭 So What Should We Do?
There are no easy answers.
Drug development is too slow
Drug pricing is too high
Medical innovation is too important
But one thing is clear:
Rushing science rarely ends well.
Montana’s experiment might look like freedom.
But without guardrails, freedom becomes chaos.
And in medicine, chaos costs lives.
We need:
Faster, smarter trial designs
Stronger oversight for compounders and “wellness” clinics
More transparency around how these treatments are marketed
A public that understands why the FDA exists—and why it isn’t just red tape

Final Dose
Science is slow for a reason.
Every miracle drug was once a risky experiment.
Every pill that saves your life went through thousands of failures first.
Yes, we can—and should—debate how much money Big Pharma makes.
But the one thing we can’t afford is to pretend that drug development is easy…
or that skipping the process is safe.
Because once we start treating medicine like tech—move fast, break things—
Well... people get broken.
As healthcare shifts towards more freedom of patient choice without restrictions on advertising and safety, it’s more important than ever for patients to be educated on the truth.
Keep looking out for yourself. And never forget that Healthcare is a Business.
See y’all at the saloon.

Alec Wade Ginsberg, PharmD, RPh
4th-Gen Pharmacist | Owner & COO, C.O. Bigelow
Founder, Drugstore Cowboy